Medical equipment plays a pivotal role in modern healthcare,
aiding in the diagnosis, treatment, and management of various
medical conditions. The reliability and safety of these devices
are paramount, as the consequences of equipment malfunction can
be life-threatening. Unfortunately, no matter how rigorous the
testing and quality control are, there is always a possibility
of defects or issues arising after a product has been introduced
into the market. This is where medical equipment recalls come into
play. In this article, we will explore the best practices for navigating
medical equipment recalls and provide valuable resources for healthcare
professionals and consumers alike.
Understanding Medical Equipment Recalls
A medical equipment recall is a process by which a manufacturer or distributor
takes corrective action to address a problem with a medical device. These
problems can range from design flaws and manufacturing defects to labeling
errors and software glitches. Recalls can be initiated voluntarily by the
manufacturer or mandated by regulatory authorities, such as the U.S. Food and
Drug Administration (FDA). A recall aims to remove or correct devices that pose
a risk to patient health.
The Importance of Early Detection
Timely detection of potential issues with medical equipment is crucial for patient
safety. This responsibility often falls on healthcare providers and facilities that
use these devices. It's essential to have robust systems in place for identifying
and reporting any problems. This includes monitoring devices for unusual behavior,
listening to feedback from patients, and staying informed about recalls and safety alerts.
‘A proactive approach that involves staying informed, establishing dedicated recall
teams, and maintaining meticulous documentation’
Best Practices for Navigating Medical Equipment Recalls
Navigating medical equipment recalls effectively requires a proactive approach and
adherence to best practices.
Stay Informed
The first step in handling medical equipment recalls is to stay informed. Manufacturers,
regulatory agencies, and healthcare organizations regularly issue alerts and notifications
about recalls. Healthcare professionals should subscribe to relevant newsletters, follow
regulatory agency websites, and use databases like the FDA's Medical Device Recalls database
to stay updated.
Establish a Recall Team
Every healthcare facility should have a designated recall team responsible for managing recalls.
This team should include members from different departments, including clinical, procurement,
and risk management. Clear roles and responsibilities should be defined, and team members should
be trained in recall procedures.
Identify Affected Devices
Once a recall notice is received, the recall team must identify all affected devices within their
inventory. This may require cross-referencing serial numbers, lot numbers, and other identifiers
provided in the recall notice. Devices that match the recall criteria should be isolated and taken
out of service immediately.
Notify Relevant Parties
Effective communication is critical during a recall. The recall team should notify relevant parties,
including staff members who use the affected equipment, as well as patients who may have been treated
with the device. Transparent and timely communication helps prevent potential harm and build trust.
Replace or Repair
Depending on the nature of the recall and the instructions provided by the manufacturer or regulatory
agency, affected devices should either be replaced, repaired or returned to the manufacturer. It's
essential to follow the recommended actions precisely to ensure patient safety.
Document Everything
Maintaining detailed records of the recall process is essential for accountability and compliance.
Document actions taken, communications sent, and any patient outcomes related to the recall. This
documentation can be invaluable in case of audits or legal inquiries.
Monitor and Audit
After the recall process is complete, it's important to continue monitoring affected devices and
conduct periodic audits to ensure compliance. This ongoing diligence helps prevent future issues and
demonstrates a commitment to patient safety.
Educate Staff
Educating healthcare staff about the importance of recalls, how to identify affected devices, and
what steps to take in case of a recall is crucial. Regular training sessions can help ensure that
everyone is prepared to respond effectively.
Resources for Navigating Medical Equipment Recalls
Having access to reliable resources is key to navigating medical equipment recalls successfully.
Here are some valuable resources for healthcare professionals and consumers
FDA's Medical Device Recalls Database
The FDA's Medical Device Recalls database is a comprehensive source of information on
medical device recalls in the United States. It provides details about the recalled
products, reasons for the recall, and actions to be taken. Healthcare professionals
can search for specific recalls and sign up for email alerts.
Manufacturer's Instructions for Use (IFU)
The manufacturer's IFU often contains important information about the correct use
and maintenance of medical devices. It may also include instructions on what to do
in the event of a recall. Healthcare facilities should have easy access to these
documents and ensure that staff members are familiar with their contents.
Healthcare Associations and Organizations
Professional healthcare associations and organizations often guide handling medical
equipment recalls. These resources can include best practices, templates for
recall plans, and educational materials. For example, the American Hospital
Association (AHA) offers resources on its website related to medical device recalls.
Patient Safety Organizations
Patient safety organizations are valuable resources for consumers who want to stay informed
about medical equipment recalls. These organizations often provide information on recalls
and offer guidance on what patients should do if they suspect they have received care
involving a recalled device.
Regulatory Agencies
Regulatory agencies, such as the FDA in the United States, Health Canada, and the European
Medicines Agency (EMA), maintain websites with information on medical device recalls and safety alerts.
These agencies are responsible for overseeing the safety and effectiveness of medical devices in their
respective regions.
‘A dynamic solution for healthcare facilities, facilitating resource mobilization, specialized expertise,
flexibility, end-to-end management, and data-driven decision-making’
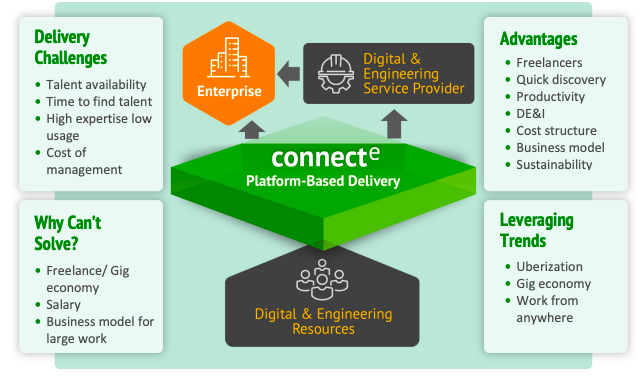
How ConnectE can help?
ConnectE can play a crucial role in Recall Management for Medical Equipment by leveraging its intelligent
workforce management platform. Here's how.
Resource Mobilization
ConnectE's On-Demand solution enables healthcare facilities to quickly source technical professionals
with expertise in medical equipment. When a recall occurs, having immediate access to qualified
professionals can expedite the assessment and mitigation process.
Specialized Expertise
Medical equipment recalls often require specific technical knowledge. ConnectE's network
of technical professionals spans various domains, including healthcare technology. This
ensures that you can connect with experts who understand the intricacies of the equipment
in question.
Flexibility
ConnectE offers a flexible workforce, ready to deploy anytime and anywhere.
In the event of a recall, you can scale your technical workforce up or down as needed,
ensuring that you have the right talent when and where it's required.
End-to-end Management
From requisition to payment, ConnectE provides an integrated platform for managing your
technical workforce. This streamlined process includes performance management, making it easier
to monitor and evaluate professionals involved in recall management.
Smart Workforce Solutions through Data-Driven Decision-Making
ConnectE’s platform leverages the power of Artificial Intelligence to match technical
professionals (TechPro) with precision, taking into account crucial factors such as
project performance, skillset proficiency, extensive experience, and cost-effectiveness.
Furthermore, ConnectE offers comprehensive Project and Financial management analytics,
placing actionable insights right at your fingertips. With ConnectE, you can harness the
full potential of data to make informed choices, optimize your workforce, and ensure the
success of your projects.
Conclusion
Navigating medical equipment recalls is a critical aspect of healthcare, and ensuring patient
safety remains a top priority. Healthcare professionals and facilities must adopt best practices
for detecting, responding to, and documenting recalls. Staying informed through reliable resources
is essential for effective recall management. By following these practices and utilizing available
resources, the healthcare industry can minimize the risks associated with medical equipment recalls
and continue to provide safe and high-quality patient care. Remember, in the world of healthcare,
vigilance and preparedness can save lives.